Gluten Should Be Labeled as a Major Food Allergen in the U.S., Just Like It Is in 87 Other Countries
- Jon Bari

- Jul 8, 2021
- 35 min read
Updated: Jan 16, 2025

"Eating without fear is our hope. Food insecurity happens everyday for Celiacs because of the constant threat of cross contact with Gluten, 80% of foods have Gluten in them, and Gluten is not required to be labeled on packaged foods in the U.S."
-- Jax Bari (age 8)
"Although the FALCPA mandates that wheat be disclosed on food labels, other grains [containing Gluten] toxic to individuals with Celiac Disease (e.g., rye and barley) need not be disclosed in plain English or when present in spices, flavorings, colors, or additives." -- Laura Derr Sim, Esq., author of "When food is poison: the history, consequences, and limitations of the Food Allergen Labeling and Consumer Protection Act of 2004"
"What is food for one is poison for another." -- U.S. Senator Royal Samuel Copeland, M.D., 1923-1938, (D, NY)
Overview
In the spirit of health equity, social justice, equal protection under the law and the Human Right to Adequate Food, it is time for the Food Allergen Labeling and Consumer Protection Act (FALCPA) to be amended to require that Grains Containing Gluten (i.e., Wheat, Barley, Rye or Oats, collectively "Gluten") be labeled on all packaged foods in the U.S., just like it is in 85 countries worldwide (see list below), including across Europe and in Canada. This amendment will be accordance with the legislative intent and the original language for the FALCPA. By requiring that Gluten be labeled on all packaged foods in the U.S., this will better protect an estimated 23 million Americans including: 3.3 million Americans who suffer from Celiac Disease (including my 8 year old son Jax) and 20 million Americans who have Non-Celiac Gluten Sensitivity. Celiac is a potentially life-threatening food allergy and auto-immune disease.
Models to Emulate: Canada, European Union and United Kingdom
The FALCPA was an important first step in providing national recognition of food allergies and Celiac Disease. Additionally, the FALCPA improved the quality of life for those living with food allergies, Celiac Disease and Non-Celiac Gluten Sensitivity. However, the FALCPA has left much on the table that is needed to protect those who must avoid ingesting Gluten for medical reasons because it is poison to their bodies. To that end, as part of amending the FALCPA to require labeling of Grains Containing Gluten, it is instructive for the U.S. Congress to see how Gluten is required to be labeled in 85 countries around world, including:
Canada - "In Canada, common allergens and gluten sources must always be clearly declared on food labels when present as ingredients or components of ingredients. They will appear in the ingredient list or in a 'Contains' statement located immediately after the ingredient list."
European Union - There are 14 allergens recognized as the most common and potent causes of food allergies and intolerances across Europe including "Cereals containing gluten: namely wheat (including specific varieties like spelt and Khorasan), rye, barley, oats and their hybridised strains) and products thereof."
United Kingdom - "In the UK, food businesses must inform you under food law if they use any of the 14 allergens as ingredients in the food and drink they provide. This list has been identified by food law as the most potent and prevalent allergens. The 14 allergens are: celery, cereals containing gluten (such as [wheat, rye], barley and oats), crustaceans (such as prawns, crabs and lobsters), eggs, fish, lupin, milk, molluscs (such as mussels and oysters), mustard, peanuts, sesame, soybeans, sulphur dioxide and sulphites (at a concentration of more than ten parts per million) and tree nuts (such as almonds, hazelnuts, walnuts, brazil nuts, cashews, pecans, pistachios and macadamia nuts)."
Across Europe, since 2005, if Wheat, Barley, Rye or Oats are used in a product, even if any of these ingredients are only used in "tiny amounts", then those ingredients must be listed on the food product's labels. Coeliac UK has produced a great video explaining the EU's consumer protection laws with food labeling. ("Coeliac" is the Greek spelling of Celiac which is used in some parts of the world.)
Labeling Gluten Aligns with Conclusions from International Food Safety Authorities & Experts, Including from the FDA
Labeling Gluten on all packaged foods in the US is in alignment with the previous conclusions of international food safety authorities and expert committees comprised of scientists, regulators, physicians, clinicians and risk managers from academia, government and the food industry including:
Joint Food and Agriculture Organization of the United Nations ("FAO")/World Health Organization ("WHO") Expert Committee on Food Additives. Evaluation of certain food additives and contaminants: fifty-third report of the Joint FAO/WHO Expert Committee on Food Additives. 2000. WHO Technical Report Series 896. World Health Organization, Geneva (“1999 FAO/WHO Expert Consultation”; also referred to as the “1999 Codex criteria”).
Food and Agriculture Organization of the United Nations/World Health Organization. “Summary report of the Ad hoc Joint FAO/WHO Expert Consultation on Risk Assessment of Food Allergens. Part 1: Review and validation of Codex priority allergen list through risk assessment.” 2021 (“2021 FAO/WHO Expert Consultation”).

The 2021 FAO/WHO Expert Consultation, which was an authoritative body chaired by the FDA’s Dr. Lauren Jackson, Chief, Process Engineering Branch, Division of Processing Science & Technology, Institute for Food Safety & Health, determined:
“Based on systematic and thorough assessments which used all three criteria (prevalence, severity and potency), the Committee recommended that the following should be listed as priority allergens: Cereals containing gluten (i.e., wheat and other Triticum species, rye and other Secale species, barley and other Hordeum species and their hybridized strains), crustacea, eggs, fish, milk, peanuts, sesame, specific tree nuts (almond, cashew, hazelnut, pecan, pistachio and walnut)." (emphasis added)
Labeling Gluten on Packaged Foods is Commensurate with the Food Industry's Existing Global Operations
Given that Gluten is required to be labeled on packaged foods in 87 countries worldwide, many of the multinational consumer-packaged food manufacturers already label Gluten on their products sold in those 87 other countries. As such, labeling Gluten is in the United States would be commensurate with their existing global operations and best practices.
To gain a better understanding of the support from the global food industry for the mandatory declaration of Gluten on all food labels, it is instructive to review the following best practice guidance from the Food and Drink Federation (“FDF”) in the United Kingdom entitled, "Gluten Labelling Guidance: Best Practice for Prepacked Foods which Include or Exclude Cereals Containing Gluten (“Gluten Labeling Guidance”)." The FDF is "the voice of the UK food and drink industry, the largest manufacturing sector in the country," and the FDF’s Gluten Labeling Guidance was published in June 2019 in partnership with Coeliac UK, Anaphylaxis Campaign, BRC (British Retail Consortium), and GFIA (Gluten Free Industry Association).
"The Food and Drink Federation (FDF) is the voice of the UK food and drink industry, the largest manufacturing sector in the country. We communicate our industry's values and concerns to Government, regulators, consumers and the media. We also work in partnership with key players in the food chain to ensure our food is safe and that consumers can have trust in it."
According to Heather Hancock, Chairman of the Food Standards Agency in the United Kingdom (the FDA’s comparable agency), "The Food Standards Agency welcomes the FDF's [Food and Drink Federation] work to achieve greater consistency in how the presence of cereals containing gluten and gluten-free claims are labelled on prepacked foods. Having a trusted consistent approach will make it easier for people with coeliac disease or with allergies to these cereals to find and understand the labelling information they need. And that means they can make safer food choices. I am very pleased to see further progress in this important area of public health and consumer protection." (emphasis added)
The Legislative Intent of FALCPA Required Labeling of Gluten on All Packaged Foods in the U.S.
In order to look forward, we must first look back. In doing so, I relied heavily on "When food is poison: the history, consequences, and limitations of the Food Allergen Labeling and Consumer Protection Act of 2004" by Laura E. Derr, as published in the Food and Drug Law Journal in 2006 ("When Food is Poison").
On May 9, 2002, Senator Ted Kennedy (D, Massachusetts) addressed the U.S. Senate:
"American families deserve to feel confident about the safety of the food on their tables... The Food Allergen Consumer Production Act will require that food ingredient statements on food packages identify in common language when an ingredient, including a flavoring, coloring, or other additive, is itself, or is derived from, one of the eight main food allergens, or from grains containing Gluten."
The clear legislative intent of the FALCPA was to protect those consumers with food allergies and Celiac Disease, both of which involve abnormal immunological responses to proteins in food (which will be further discussed in the section below "Biology Basics"). The requirement that Gluten be labeled on all packaged foods in the U.S. was expressly contemplated in 2002 in the original versions of the FALCPA:
S. 2499 - "The Food Allergen Consumer Protection Act of 2002," as introduced by Senator Kennedy [and Senator Clinton (D, New York)], would require products to list in bold face type the common name any of the eight major food allergens (milk, egg, fish, crustacea, peanuts, tree nuts and soybean), proteins derived from those substances and other Glutens such as rye, barley, oats, and triticale," according to Inside Health Policy's FDA Week, August 9, 2002 (page 6). (emphasis added) "For purposes of this Act, the term 'known food allergen' means any of the following: A) Milk, Egg, Fish, Crustacea, Tree nuts, Wheat, Peanuts, and Soybeans... C) Other Grains containing Gluten (Rye, Barley, Oats, and Triticale)."
H.R. 4704 - The "Food Allergen Consumer Protection Act," as introduced by Representative Nita Lowey (D, NY-18) in 2002, expressly included "Grains Containing Gluten" among the "allergens" within the scope of the legislation's mandatory labeling scheme. H.R. 4704, 107th Cong. [Section] 3 (2d Sess. 2002). In addition to Wheat, Grains Containing Gluten included Rye, Barley, Oats, and Triticale.
According to When Food is Poison, which was published in 2006, “The drafting and passage of the FALCPA [2002-2004] was accomplished because the atmosphere in America surrounding food labeling has shifted dramatically over the past several decades. This altered culture is due to the convergence of numerous factors, including scientific advances in diagnosing food sensitivities and detecting allergens in foods, increased public awareness about food sensitivities, the mobilization of people with food sensitivities, the prioritization of food allergy issues by FDA, and recognition by industry of the growing market for products to meet special dietary needs.” (Page 94)
What is Food Allergy? The Similarities and Differences Between Non-IgE-Mediated Mechanisms with Celiac Disease & Typical IgE-Mediated Mechanisms

The "Watered Down" FALCPA
While the FALCPA was being debated in Congress, the food industry lobbied against the FALCPA in its entirety and insisted that voluntary labeling of food allergens was sufficient. However, the food industry seemingly evolved its position and recognized that there was a convergence of factors, a perfect storm, that finally gave Congress the appetite and resolve to pass the FALCPA. With that recognition, the food industry then demanded certain compromises and concessions.
On August 9, 2002, the FDA Week reported that Senator Kennedy floated an amendment to the FALCPA,
"But the food industry source says that even though the amendment goes in the right direction, the food industry continues to oppose the bill on the grounds that a mandatory approach is not appropriate or needed, and that instead the food industry should be allowed to continue to implement its voluntary guidelines... Critics of the bill [in the food industry] had charged that scientifically, Gluten is an intolerance not an allergen."
On September 20, 2002, the Center for Science in the Public Interest (CPSI) reported that S.2499 had become "stymied" in the Senate Health, Education, Labor and Pensions (HELP) Committee which was chaired by Senator Kennedy,
"Senate Democrats and Republicans have been working on compromise language to move the legislation out of the Health, Education, Labor, and Pension committee, which Senator Kennedy chairs. Even though Democrats have offered significant concessions, key Republicans on the committee have sided with the food industry, which generally opposes any labeling changes, according to CSPI."
"Most of the groups involved in advocating for the FALCPA were food allergy groups [such as FAAN, the Food Allergy & Anaphylaxis Network that had been working on food allergy labeling for 20 years]. In contrast, ACTF [American Celiac Task Force, which later became the American Celiac Disease Alliance], which was composed of leaders of national Celiac Disease support groups, medical professionals, research institutions, and representatives of gluten-free food manufacturers, represented individuals with Celiac Disease. ACTF acted as the voice of the celiac community during the push to pass the FALCPA. It was formed in 2003 after the Celiac Disease prevalence study results were released by the University of Maryland Center for Celiac Research. This study 'opened the doors for people paying much more attention' to Celiac Disease, according to ACTF Co-chair Andrea Levario... 'This was a true grassroots effort,' Levario says. 'Within the span of 18 months, ACTF was created, it mounted a lobbying effort, and it witnessed the successful passage of the FALCPA. ACTF 'has no money. This was all done strictly via word of mouth, via the Internet, via e-mail... We were a voice in all this, and it was strictly, totally grassroots, every step of the way, which is pretty phenomenal.'" (When Food is Poison, page 102, Footnote 217).
In contrast to the years of advocacy by the Food Allergy & Anaphylaxis Network, it was not until April 27, 2004 that individuals with Celiac Disease testified for the first time before a Congressional Committee. According to Allison Herwitt, former Co-Chair, Legislative Project, American Celiac Task Force (ACTF),
"Lisa Murphy, and her son, Colin, represented the ACTF before the House Appropriations Subcommittee on Labor, HHS, and Education... The Labor-HHS Subcommittee determines how much money NIH receives each year. Having individuals with Celiac Disease provide information about the disease is critical to securing funding for research. After hearing the testimony, Subcommittee Chairman, Ralph Regula (R-OH), asked if food labels were a problem for Celiacs. Not missing a beat, Lisa offered an emphatic, Yes, then highlighted problems she has encountered. Rep. Nita Lowey (D-NY), sponsor of H.R. 3684, the Food Allergen Labeling and Consumer Protection Act, and member of the Subcommittee, explained the bill was drafted to help individuals like Lisa, and Colin."

"At least one of the three national celiac disease support groups, the Celiac Sprue Association (CSA), supported the FALCPA but advocated for the further labeling of all gluten-containing source ingredients. [CSA is now part of the National Celiac Association.] A provision in a 2002 bill that called for the inclusion of all gluten-containing grains in an allergen labeling scheme was dropped as a part of compromises reached while the bill was in committee." (When Food is Poison, page 142, Footnote 434).
Unfortunately, the pleas of the Celiac Sprue Association and others -- that the FALCPA's original language remain which required that Gluten be labeled -- were ignored. Their thought leadership and efforts at the time could not compete with the well funded lobbying efforts of the food industry looking for any negotiated concessions (wins) to the FALCPA. In spite of Gluten disclosures being required on all food labels in the original bills and their respective amendments, some critics in the food industry were able to exert their influence on FALCPA's final language as part of a grand bargain. According to Inside Health Policy's "FDA Week", September 27, 2002 (page 5),
"Substitute [bill] pulls mandatory Gluten declaration... This week the Senate Health Committee passed without objection a watered-down version of a bill that would require food processors to label the eight most common types of food allergens in plain English. The substitute version, unlike the underlying bill... would not require the declaration of Gluten."
According to When Food is Poison, "Senator Kennedy introduced sister bills to those introduced in the House and helped work out a compromise amendment that was critical to the enactment of the FALCPA." (page 110) The subsequent compromise bill served as the basis of S. 741, the FALCPA.
As a result, unlike the mandatory FALCPA labeling scheme for the Big 8 food allergens in the U.S., Gluten Free labeling is permissive (voluntary), in the U.S.
In other words, the information strongly suggests that the Celiac community took one for the proverbial team -- the food allergy community -- when the language for the labeling of Gluten was revised from mandatory to voluntary in order to get the FALCPA passed which required labeling of the Big 8 food allergens.
In addition to Senator Jeff Sessions (R, Alabama) sponsoring S.741, there was broad bipartisan support for the FALCPA in the Senate with a total of 21 Co-Sponsors (10 Democrats and 11 Republicans) including: Senators Wayne Allard (R, Colorado), Jeff Bingaman (D, New Mexico), Susan Collins (R, Maine), Larry Craig (R, Idaho), Mike Crapo (R, Idaho), John Ensign (R, Nevada), Judd Gregg (R, New Hampshire), Tom Harkin (D, Iowa), Blanche Lincoln (D, Arkansas), Zell Miller (D, Georgia), Richard Shelby (R, Alabama), Pete Domenici (R, New Mexico), Mike Enzi (R, Wyoming), Gordon Smith (R, Oregon), Patty Murray (D, Washington), Orrin Hatch (R, Utah), Mary Landrieu (D, Louisiana), Ron Wyden, D, Oregon), Mark Pryor (D, Arizona), Dick Durbin (D, Illinois), and Maria Cantwell (D, Washington).
Food allergies and Celiac Disease are bipartisan issues since these health issues impact all Americans from every state, from every political party, from every political ideology, etc.
Organizations Which Lobbied Congress on Food Labeling
According to Open Secrets, there were three unique organizations that registered to lobby on S.741: Food Allergen Labeling and Consumer Protection Act of 2004 including: the American Farm Bureau, the Center for Science in the Public Interest and the American Bakers Association (ABA). The ABA touts itself as the "voice of the baking industry since 1897" for its 300+ members, and according to Paul C. Abenante, ABA President & CEO,
"ABA is a voluntary trade association dedicated to representing the interests of the wholesale baking industry before the United States Congress, federal agencies, state legislatures, and international regulatory authorities. ABA tackles key issues facing the grain-based foods industry and initiates key reforms to make positive impacts on the industry. ABA represents approximately 80% of the wholesale bakeries in the U.S. as well as their suppliers...In effect, ABA provides a comprehensive resource to the baking industry that can address most, if not all, sectors of the grain-based foods industry, operations and activities." (emphasis added, posted on April 17, 2004)
FALCPA Fell Short in Safeguarding Celiacs
Since January 1, 2006, FALCPA has required that the top 8 Major Food Allergens must be labeled on all food products in the U.S.

However, the Celiac community had to wait a full decade for any type of voluntary FALCPA labeling protections to be enacted. According to "When food is poison: the history, consequences, and limitations of the Food Allergen Labeling and Consumer Protection Act of 2004":
"The FALCPA contains a number of provisions designed to address food allergen safety concerns not met by the labeling requirements and to improve scientific, medical, and public knowledge about food allergies. The FALCPA directs that within two years after its enactment, the Secretary [of Health and Human Services] must issue a proposed rule to define and permit voluntary use of the term 'gluten-free' on food labels. The FALCPA directs FDA to issue a final rule by August 2008. The House Committee Report (H.R. Rep. No. 108-608 at 18) on the FALCPA states that, '[g]iven the devastating nature of Celiac Disease, the Committee urges the Secretary to move expeditiously in implementing the requirements of this section.' Currently, there is no standard definition of 'Gluten Free' in the United States and studies have found that some products proclaiming themselves 'Gluten Free' may contain Gluten. The FALCPA calls for the Secretary to consult with experts and stakeholders when drafting the rule." (emphasis added; page 119)
“While this provision to define ‘Gluten Free’ is a significant step in the right direction, use of this declaration by manufacturers merely is voluntary rather than mandatory. Although people with Celiac Disease will be able to trust 'Gluten Free’ declarations beginning in 2008 [which did not occur until 2014], the degree to which safety and convenience will improve for those with Celiac Disease depends on how widespread voluntary usage of the Gluten Free claim becomes. Earlier legislative allergen labeling efforts included provisions requiring the Secretary of HHS to assess, after a 'Gluten Free’ standard is defined, whether additional labeling of Gluten is necessary on the label. [S. 3001 and H.R. 5747, 107th Cong. Section 6(d) (2d Sess. 2002); see also S. 2499, 107th Cong. Section 6(d) (2d Sess. 2002); S. Rep. No. 107-322, at 8 (accompanying S. 2499, as reported in Senate).] This provision was cut from the FALCPA.” (emphasis added; page 144)
"The clear labeling of wheat under the FALCPA, in fact, may have the perverse effect of harming those who must avoid Gluten. A Gluten Free product always is wheat-free, but the reverse is not true. Children or caregivers of children with Celiac Disease may assume incorrectly that a wheat-free product is Gluten Free if they are not familiar with or do not remember the various terms for gluten-containing ingredients (e.g., rye, barley, and malt) besides wheat... It is clear, however, that including Gluten Containing Grains besides wheat in the FALCPA's allergen labeling scheme, expressly requiring FDA to consider Celiac Disease in its ingredient exemption decisions, and requiring the 'Gluten Free' declaration on products without Gluten could have gone significantly further to assist people living with this [Celiac] Disease." (emphasis added; pages 143-145)
According to the FDA, "[o]n August 2, 2013, FDA issued a final rule defining 'Gluten-free' for food labeling, which is helping consumers, especially those living with celiac disease, be confident that items labeled “gluten-free” meet a defined standard for gluten content. 'Gluten-free' is a voluntary claim that can be used by food manufacturers on food labels if they meet all the requirements of the regulations."
The final rule was implemented as of August 5, 2014 for voluntary labeling of "Gluten Free" claims by manufacturers.
Celiacs Got Half a Loaf at Twice the Price
The FALCPA was signed into law by President George W. Bush on August 2, 2004 and mandatory labeling of the top 8 allergens began on January 1, 2006 (Public Law No: 108-282). However, it took a decade to implement voluntary labeling of Gluten Free products on August 5, 2014.
"I am frustrated that, a decade since the passage of the Food Allergen Labeling and Consumer Protection Act, the FDA has yet to lay out clear standards for the regulation of Gluten Free labeling." -- Rep. Nita Lowey, July 15, 2013
Unlike the FALCPA labeling scheme for the top 8 major food allergens in the U.S., Gluten Free labeling is permissive, not mandatory in the U.S. In other words, whereas sufferers of the current top 8 major food allergens rely on what ingredients are included in required labeling disclosures of packaged foods, Celiacs must rely only on what ingredients are excluded in voluntary Gluten Free labeling disclosures on packaged foods.
Think of it like this. If someone has a nut allergy, that person can rely on a food manufacturer being required to label whether a food product has nuts in it.
If someone has Celiac Disease, that person cannot rely on food manufacturers labeling Gluten in a food product. Instead, Celiacs must rely on what ingredients are not included in voluntary Gluten Free labeling on food products. That is not very safe and it does not seem very fair.
In addition to having to wait a full decade after FALCPA was passed for any help with regard to labeling, mandatory Gluten labeling with FALCPA got the short end of the legislative negotiating stick in at least several respects (in no order of importance):
Second Class Food Allergen - As a result of FALCPA, the top 8 Major Food Allergens were required to be labeled on all packaged foods in the U.S. since January 1, 2006. However, as a result of FALCPA, guidelines for the voluntary labeling of Gluten Free products only went into effect as of August 5, 2014. In my opinion, that has lead to all sorts of challenges and limitations including perpetuating a Gluten as a "Second Class Food Allergen", one that is not taken as seriously as other allergens/poisons (the brunt of too many jokes including from Jimmy Kimmel), and in turn the only treatment for Celiac has been subject to an implicit bias by far too many as a fad diet and not a serious disease.
Food Insecurity - I also believe that FALCPA has had the unintended and perverse consequence of contributing to food insecurity which is the everyday life of Celiac patients, no matter the person's age or socio-economic status. Additionally, I believe that FALCPA's labeling scheme has made Gluten Free foods cost so much more than a regular market basket of food (the "Gluten Free tax"). The treatment burden for Celiac Disease is compounded by the increased cost of a “market basket” of Gluten Free products against a comparable market basket of Gluten containing products whereby research indicates that Gluten Free products were 183% more expensive nationwide.
Medicine Insecurity - The FALCPA does not extend to prescription drugs making it nearly impossible to determine the presence of Gluten in prescription medicine. Nonetheless, the FALCPA's unequal treatment has also left those with hypersensitivities to Gluten vulnerable with undermining their right to know whether Gluten is in various prescription medicines prescribed for a plurality of incremental health reasons that those living with Celiac may also be experiencing. After repeated efforts to shed light on Gluten in medicine, the FDA initiated a draft guidance in 2017 (which appears to have not been finalized), encouraging drug manufacturers to disclose the presence of Gluten. While some manufacturers have taken this step, many manufacturers have not. This leads to anxiety of not knowing whether or not medication is doing more harm than good. In my opinion, if we had equal treatment under FALCPA for food that is required everyday, then the Celiac community would have a better chance of getting medicines also labeled. In other words, how can we expect to get medicine labeled, which is something many Celiacs ingest occasionally, if we cannot require food labeled for Gluten which is ingested multiple times daily to survive?
Medical Research Inequity - Federal research funding from the NIH of Celiac Disease has been historically inadequate in comparison with other digestive diseases and autoimmune diseases. This was the subject of a bipartisan Congressional Briefing held by Representatives Dwight Evans (D, PA- 3) and John Joyce, M.D. (R, PA-13) in January 2020. My premise is that if FALCPA had required mandatory labeling of Gluten that the Celiac ecosystem would have benefited in myriad ways including with regard to overcoming what appears to be an implicit boas by the NIH that a Gluten Free diet is all that is needed as compared to all that has ever been available. It is instructive to read the peer reviewed academic analysis published in 2017 by the American Gastroenterological Association entitled, “Disparities Among Gastrointestinal Disorders in Research Funding From the National Institutes of Health.” This analysis was written by some of the world’s thought leaders in GI research, which found that, out of various Gastrointestinal Disorders, from 2011-2015:
A. “Celiac disease consistently received the lowest amount of NIH funding over the 5-year period, at approximately $3 million per year.” NIH has spent about $1.00 per American with Celiac per year, and that is way too little to move the needle.
B. “Celiac disease consistently received the lowest amount of NIH grants, at approximately eight grants per year.”
C. “In conclusion, NIH funding of GI diseases is not proportional to disease prevalence or mortality.” (emphasis added)
In addition to the high cost of Gluten Free food, I believe that that the FALCPA has contributed to an implicit bias against those who medically require a strict lifetime Gluten free diet in several respects including limited calls to action to protect those who medically cannot eat foods with Grains Containing Gluten, including from the Food & Drug Administration, National Institutes of Health, Department of Defense, U.S. Department of Agriculture, etc.
The Right to Know About Gluten Ingredients - Food is Our Only Medicine
On October 3, 2011, Andrea Levario, Executive Director, American Celiac Disease Alliance, captured the essence of the needs of the Celiac community in a letter to the FDA, "There is no medical intervention for the treatment of celiac disease, no drug, no ongoing therapy. The treatment, while medically prescribed, is self-administered and in many instances without medical oversight. Gluten-free foods, in all forms, are the equivalent of a prescription medication used to manage another lifelong, chronic condition."
Cautionary Tale on the Dangers of Grains Containing Gluten
In December 2020, Hillary Carter, food allergy mom and advocate, bravely shared her family's harrowing ordeal. "When 'Safe' Food Isn’t… Our Applegate Anaphylaxis Story" is about her son Grayson who ate chicken nuggets that were labeled Gluten Free and casein-free, in addition to free of the Top 8 allergens per the manufacturer's Web site. However, after eating the nuggets, Grayson went into anaphylactic shock because the product somehow contained traces of Gluten.
Thankfully, after receiving 4 doses of Epi and enduring an overnight hospital stay, Grayson recovered. However, I cannot help but think that this happened to a young boy from eating a product that was labeled Gluten Free that somehow got cross contaminated with Gluten. While this is a cautionary tale over the real risks of cross contamination, it is also an important cautionary tale of why mandatory labeling of Gluten must now be enacted because of all of the places where Gluten can hide in plain sight and be dangerous to those who consume even trace amounts of Gluten, regardless of what biologically happens in the body (i.e., allergic or auto-immune response).
Celiac Disease Can Be Just as Grave and Deadly as Food Allergies
On July 20, 2004, Representative Lowey, the primary House sponsor of FALCPA, spoke on the House floor and urged her colleagues to pass FALCPA,
"Navigating insufficient labels is much more than an irritation for the millions with food allergies. It is a matter of life and death. Unfortunately, the situation is the same for those with Celiac Disease, a lifelong digestive disorder that damages the small intestine and interferes with absorption of nutrients from food. Although Celiac sufferers do not go into anaphylactic shock if they consume Gluten, the consequences of leaving the disease undiagnosed or untreated can be just as grave and deadly, potentially leading to additional autoimmune disorders, infertility, osteoporosis or cancer."
As discussed, in seeking concessions from lawmakers back in 2004, the well funded food industry lobbied Congress that Celiac Disease should not be viewed in the same light as food allergies (i.e., peanuts, tree nuts) since Celiacs do not go into anaphylactic shock from Gluten ingestion. However, there are those like Grayson who are allergic to Grains Containing Gluten and can suffer a severe allergic reaction from even a trace ingestion of Gluten.
Additionally, the argument from the food industry circa 2002-2004 that Celiacs do not go into anaphylactic shock from Gluten ingestion is a red herring. That is as uninformed as saying that a concussion protocol should not apply to a person who suffers a head injury, but who does not become unconscious. Today, we treat all head trauma seriously since there can be critical short and long-term consequences from not implementing a concussion protocol, even if the immediate impact of the head trauma is not visible. In other words, after being exposed to Gluten, Celiac patients such as my son can suffer both serious short-term symptoms such as diarrhea and vomiting and long-term medical complications including liver disease, inflammatory bowel disease, osteoporosis, neurological conditions, immunological scarring and cancer. That is in part why Gluten must be labeled on all packaged foods for those with Celiac Disease, Allergies to Grains Containing Gluten and Non-Celiac Gluten Sensitivity.
While I appreciate that there are scientific distinctions in the way that the body reacts to an IgE-Mediated Food Allergy (i.e., wheat allergy) vs. a Non-IgE-Mediated Food Allergy (i.e., Celiac), the bottom line is that what is food for one is poison for another, including for those with Celiac. The FALCPA should provide equal protection under the law, and it has not for those who live with Celiac, Non-Celiac Gluten Sensitivity and allergies to Grains Containing Gluten.
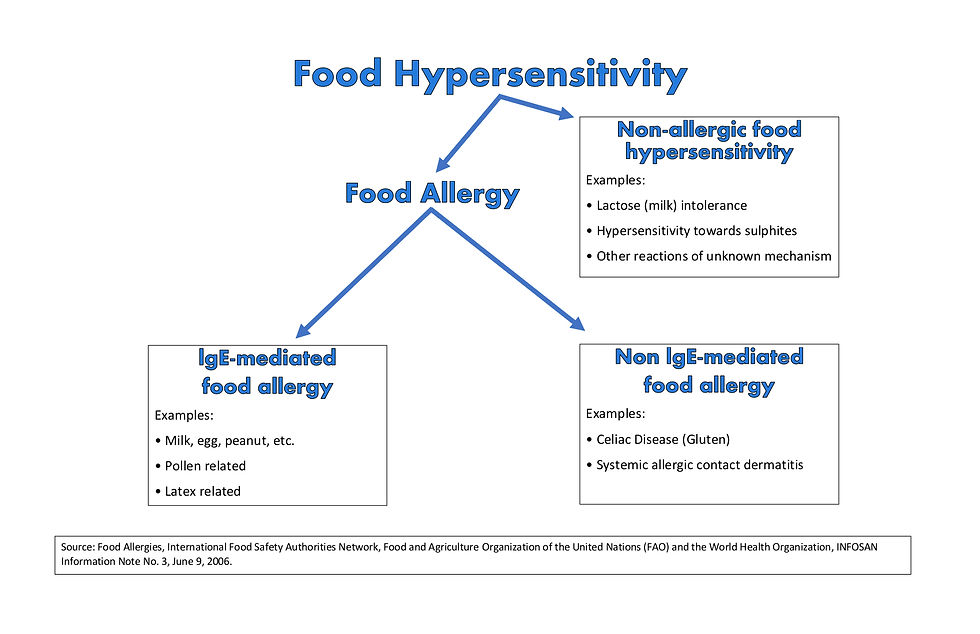
Additionally, it is instructive to realize that both IgE-Mediated Food Allergies and a Non-IgE-Mediated Food Allergy (i.e., Celiac) share many similarities with one another including that the only treatment is strict avoidance of the offending food protein, and in order to accomplish that, consumers must rely on food labels to know what is in the food that they are eating.
Oats Must Be Labeled Because Cross Contamination is "Inevitable"
As part of amending the FALCPA to require the labeling of Grains Containing Gluten, it is important to understand that Oats are considered as Grains Containing Gluten in Canada, in the UK, and across the European Union, and therefore Oats must be included on food labels in those countries.
To understand more about Oats, it is instructive to note the following "Background of the Invention" from U.S. Patent Number 9,968,937 that General Mills, Inc. was granted for a "Method for Producing Gluten-Free Oats":
"Oats themselves do not contain gluten. However, oats cultivated in North America, Europe and even other parts of the world are commonly contaminated by gluten containing foreign grains, including wheat, barley, rye and triticale. This contamination is commonly known to come from various sources, mainly from the rotation of small grain crops on the same land, with residual contaminating seeds germinating with a seeded oat crop. In addition, contamination from other grains which are harvested, transported, stored and merchandized in common with oats is a contributing factor. As a result, it is not uncommon to find from 0.5% to 5.0% of these other grains mixed with commercially marketed oats. Therefore, absent dedicating land, harvesting equipment, transporting vehicles, storage units, packaging and production facilities, and the like only for use in connection with oats, cross contamination is inevitable."
That is why S. 2499 - "The Food Allergen Consumer Protection Act of 2002," as introduced by Senator Kennedy and Senator Clinton (D, New York), and H.R. 4704 - The "Food Allergen Consumer Protection Act," as introduced by Representative Nita Lowey (D, NY-18) in 2002, each contained provisions to require that in addition to Wheat, Grains Containing Gluten included Rye, Barley, Triticale and Oats.
According to the Society for the Study of Celiac Disease, "...[A]lthough the [general] consensus is that pure (not contaminated with gluten) oats are safe for most patients with celiac disease, contamination with other cereal sources that may contain gluten, needs to be avoided."
In other words, Oats must be labeled so that consumers can make informed and safe choices as to what products contain Oats since standard Oats (also referred to as commodity Oats) may very likely have been cross contaminated with Gluten. According to Beyond Celiac, "Even though oats are naturally gluten-free, a small portion of people with celiac disease still react to them."
Manufacturers can then also voluntarily include supplemental information on the food label whether the Oats used were Gluten Free Oats that were grown, harvested and processed in a way that kept them away from other Grains Containing Gluten, and are therefore generally accepted as safe for many with Celiac Disease. Manufacturers can also then voluntarily disclose that they have scrubbed Gluten from their Oats using mechanical and optical processes.
Biology Basics
According to When Food is Poison, the FALCPA addresses both IgE-Mediated Food Allergies (i.e., Eggs, Nuts, Wheat) and Non-IgE-Mediated Food Allergies (i.e., Celiac Disease with Gluten). According to When Food is Poison,
“The blanket term 'food sensitivity' encompasses three basic types of ailments associated with adverse responses to foods that are safe for the vast majority of people to ingest -- food intolerances (e.g., lactose intolerance), immediate hypersensitivity reactions (typically known as 'food allergies'), and delayed hypersensitivity reactions (the most notable of which, for purposes of this article, is celiac disease). The three types of food sensitivities are differentiated by the specific biological mechanisms that lead to adverse reactions when a sensitive individual comes into contact with an offending food. Because the FALCPA addresses food allergies and celiac disease, this article focuses on these two ailments. The term 'food sensitivity' when it is used in the remainder of this article refers only to food allergies and celiac disease. Food allergies and celiac disease both involve abnormal immunological responses to proteins in food. When someone is allergic to a food, that person's immune system mistakenly reacts to certain proteins in the food as if the protein were an invading pathogen; the immune system responds in an exaggerated fashion to counter this perceived invasion. The most common types of food allergies (those mediated by allergen-specific immunoglobulin E, or IgE, antibodies) are known as 'immediate hypersensitivity' reactions because symptoms occur within minutes to a few hours after exposure to the allergen.
In contrast with food allergies, celiac disease involves a cell-mediated immune response against gluten (proteins found in wheat, barley, and rye). The onset of symptoms in cell-mediated food reactions, also known as delayed hypersensitivity reactions, begins within six to twenty-four hours after ingestion of the offending food, and it can take up to ninety-six hours for a reaction to subside. Ingestion of gluten is responsible for a wide variety of health dangers, including damage to intestinal lining, malnutrition, stomach pain, nausea, mental distress, migraine headaches, osteoporosis, neurological conditions, additional autoimmune disorders, and cancer. Thus, although people with celiac disease are not at risk of anaphylactic shock, the consequences of not following a strict gluten-fee diet can be 'just as grave and deadly'."
"No medicine exists that can allow people with food allergies or celiac disease to ingest the offending food; the only treatment for people with food allergies and celiac disease is to strictly avoid exposure to offending proteins." (pages 67-69)
According to the Mayo Clinic, "Food allergy symptoms usually develop within a few minutes to 2 hours after eating the offending food."
In 2019, new research showed that the body's immune system in a person with Celiac responds much quicker than previously scientifically proven. That said, Celiacs can anecdotally share their lived experiences that their body's immune system can often react quickly after they have been Glutened and various symptoms occur. If Celiac Disease patients who are following a strict Gluten Free diet "are exposed to gluten-containing food, they typically suffer from gastrointestinal reactions occurring 1 to 2 hours after the gluten exposure," according to this research, "Cytokine release and gastrointestinal symptoms after gluten challenge in celiac disease."
In addition, since the passage of the FALCPA, there have been some limited Oral Immunotherapy therapies developed which allow some people with IgE-Mediated Food Allergies to eat small amounts of offending proteins. However, for the vast majority of people with of IgE-Mediated Food Allergies, the required labeling scheme of the FALCPA has remained of paramount importance to keeping them safe.
Since it was first theorized in 1887 that treating Celiac patients “must be by means of diet” (1887 was ironically the same year that the NIH was founded), and it was discovered in 1952 that Gluten was the trigger of Celiac, the one and only Celiac treatment has remained the same -- strict adherence to a Gluten Free diet! In 2021, it is of paramount importance that Gluten be required to be labeled in the U.S. to better protect those with Celiac.
Two Parallel Universes with Mandatory & Voluntary Labeling of Gluten
Since we embarked on our family's Celiac journey when Jax was diagnosed in August 2018 at age 5 right before he started Kindergarten, I have always been perplexed at how Celiacs are not protected in the same manner in the U.S. as those with food allergies and moreover in the same manner as in 85 countries around the world including in Canada and across Europe for those with Celiac Disease, Non-Celiac Gluten Sensitivity and Allergies to Grains Containing Gluten. It seems that everyday I see some online discussions among Celiac parents and patients trying to determine if a product is safe to eat because of incomplete and inadequate labeling.
Celiac Disease and food allergies, especially for kids, takes away a level of childhood innocence and freedom. I often think about how different Jax's life would be had Grains Containing Gluten been included as one of the 8 top allergens instead of just including Wheat.
"When I learned to read five years ago in kindergarten,
I started with Dr. Seuss, Mother Goose, and ingredients labels."
-- Sarah Gitlin, age 10 (allergic to peanuts, tree nuts, and fish) FDA, Transcript: Public Meeting On: The Challenge of Labeling Food Allergens, August 13, 2001.
For my son, when he was in Kindergarten, he learned to read and started with fairy tales and food labels.
I think that Sliding Doors, the 1988 motion picture starring Gwyneth Paltrow, is an allegory for the two parallel universes with mandatory labeling of Gluten (what could have been, but for food industry lobbying against it) and voluntary labeling of Gluten (what has been the status quo since 2014 because of the food industry lobbying). Sliding Doors chronicles the life of Helen Quilley (played by Paltrow) who is a young woman living in London with two parallel universes and storylines, based on the two paths that Helen's life could take depending on whether or not she caught a London Underground train.
"When Food is Poison" has an in-depth discussion on Celiac Disease, "Because the FALCPA addresses food allergies and celiac disease, this article focuses on these two ailments." While the entire article is fascinating, I found the section "The FALCPA's Impact on People with Celiac Disease" particularly interesting (pages 142-145).
According to the Food Allergy Research and Resource Program at the University of Nebraska-Lincoln, there are 85 countries around the world which require that Gluten be labeled as a major food allergen on packaged foods. However, the United States is lagging behind in protecting people with Celiac Disease, Non-Celiac Gluten Sensitivity and Allergies to Grains containing Gluten.
It is worth reiterating that this is a matter of health equity and social justice. It is a matter of the Human Right to Adequate Food.
“Our thought has been that the purchaser of food products,
the one who is going to take those food products into his system, is entitled,
as a matter of simple right, to know what he is eating.”
Walter G. Campbell, Chief of the Food and Drug Administration (1934)
Food Allergy Allies - Labeling Sesame
Even though no one in our family has a Sesame allergy, my wife and I were honored to participate in the Food Allergy Research & Education's (FARE's) Courage at Congress event in March 2021, and I was one of many food allergy allies who advocated to Congress for Sesame labeling requirements. On April 23, 2021, President Joe Biden signed the Food Allergy Safety, Treatment, Education and Research (FASTER) Act of 2021 into law, ensuring that Sesame will be labeled on all packaged foods in plain language by January 1, 2023. The FASTER Act will protect about 1.6 million Americans who are allergic to Sesame. This marked the first time since 2004 that a new major food allergen has been added to the top 8 allergens in the U.S. as set forth in the Food Allergen Labeling and Consumer Protection Act (FALCPA). With the FASTER Act, the U.S. has joined 46 other countries around the world where Sesame must be labeled on packaged foods including in Canada and across Europe.
FASTER Act Is a Bipartisan Blueprint for Amending FALCPA And Requiring That Gluten Be Labeled
With respect to revising FALCPA to require that Gluten be labeled on all packaged foods in the U.S., just like it is in 85 countries across the world, including across Europe and in Canada, and in accordance with the legislative intent and original language for FALCPA, it is instructive to review how the FASTER Act became law.
March 3, 2021 - The FASTER Act, S. 578 was introduced by Senator Tim Scott (R, SC) along with 8 Cosponsors including Sen. Chris Murphy (D, CT), Sen. Susan Collins (R, ME), Sen, Kirsten Gillibrand (D, NY), Sen. Thom Tillis (R, NC), Sen. Richard Blumenthal (D, CT), Sen. Tammy Baldwin (D, WI), Sen. Roger Marshall (R, KS) and Sen. Raphael Warnock (D, GA). On that same day, the FASTER Act was passed by Unanimous Consent in the U.S. Senate!
April 14, 2021 - The House overwhelmingly approved the FASTER Act in a bipartisan manner in a vote of 415 voting "yea" and only 11 voting "nay." The U.S. House of Representatives passed the FASTER Act to add Sesame as 9th top allergen! This was a truly remarkable moment for the food allergy community especially this early in the 117th Session of Congress as more than 2,150 bills have already been introduced in Congress and the FASTER Act is only one of seven that has passed the Senate.
April 23, 2021 - President Joe Biden signed the FASTER Act into law which added Sesame as the 9th major allergen that is required to be labeled on packaged foods in the U.S.
Cost Estimates for the FASTER Act Are Instructive for Estimating Costs of Requiring Gluten Labeling
In order to understand the costs involved with requiring that Gluten be labeled on all packaged foods in the U.S., it is instructive to look at the Congressional Budget Office Cost Estimate for H.R. 2117, which was the predecessor to the FASTER Act. In other words, I think that the costs to the Federal government and private sector with adding Grains Containing Gluten to the definition of major allergens would be in line with the CBO's projections for the FASTER Act.
"H.R. 2117 also would add sesame to the definition of major allergens, would permit the Secretary of Health and Human Services to add food ingredients to that definition, and would require FDA to add a section to a report that the agency produces under current law. Using information from FDA, CBO expects the agency would require, on average, the equivalent of three additional full-time employees in each fiscal year from 2021-2025 to implement regulations and guidance that add sesame as a major food allergen and to evaluate whether new ingredients should be added to the list of major allergens. CBO estimates the new staffing and related expenses would cost about $5 million over the 2021-2025 period. H.R. 2117 would impose a private-sector mandate as defined in the Unfunded Mandates Reform Act (UMRA) by requiring manufacturers of food products containing sesame to include additional information on the products’ label. Because the mandate would require a minor change to existing labels, CBO estimates that the cost of the mandate would not exceed the annual private-sector threshold established in UMRA ($168 million in 2020, adjusted annually for inflation)."
85 Countries Around the World Require That Gluten Be Labeled
According to the University of Nebraska-Lincoln, the following countries require that Gluten be labeled on packaged foods: Anguilla, Antigua and Barbuda, Argentina, Australia, Austria, Bahamas, Barbados, Belarus, Belgium, Belize, Bermuda, Bolivia, Brazil, British Virgin Islands, Bulgaria, Canada, Cayman Island, Chile, China, Colombia, Costa Rica, Croatia, Cuba, Cyprus, Czech Republic, Denmark, Dominica, Egypt, El Salvador, Estonia, Fiji, Finland, France, Germany, Greece, Grenada, Guatemala, Guyana, Haiti, Honduras, Hong Kong, Hungary, India, Ireland, Italy, Jamaica, Kazakhstan, Kuwait, Latvia, Lithuania, Luxembourg, Malawi, Malaysia, Malta, Mexico, Montserrat, Morocco, Netherlands, New Zealand, Nicaragua, Oman, Philippines, Poland, Portugal, Qatar, Romania, Russia, Saint Lucia, Saudi Arabia, Singapore, Slovakia, Slovenia, Spain, St. Kitts and Nevis, St. Vin. and Grenada, Suriname, Sweden, Thailand, Trinidad and Tobago, Turkey, Turks and Caicos Island, Ukraine, United Arab Emirates, United Kingdom, Venezuela, Vietnam, and Yemen.
Conclusion
With respect to amending the FALCPA to require that Gluten be labeled on all packaged foods in the U.S., I think that there are some key catalysts/accelerants that can assist Congress in this new era of health equity and social justice for the estimated 23 million Americans who suffer from Celiac Disease and Non-Celiac Gluten Sensitivity:
the original letter and spirit of FALCPA with requiring labeling of Grains Containing Gluten,
the comparable models in 85 countries around the world including in Canada and across Europe where the labeling of Grains Containing Gluten in required, and
the recent bipartisan passage of the FASTER Act to require the labeling of Sesame to protect the 1.6 million Americans who are allergic to Sesame.
Primary Sources
"When food is poison: the history, consequences, and limitations of the Food Allergen Labeling and Consumer Protection Act of 2004", by Laura E. Derr, Food and Drug Law Journal, Vol. 61, No. 1 (2006), pages 65-165, https://www.jstor.org/stable/26660870
Inside Health Policy's FDA Week, August 9, 2002 (page 6).
Inside Health Policy's "FDA Week", September 27, 2002 (page 5).
Special Thanks to the Leaders & Legends
Thank you to both Laura Derr and Peter Barton Hutt for such meaningful work! Ms. Derr wrote the "When Food is Poison" treatise when she was a student at Harvard Law School, under the supervision of Lecturer on Law Mr. Hutt, Partner at Covington & Burling, Washington, D.C., for Harvard Law School's Winter 2005 Food and Drug Law course. When Food is Poison won First Place in the 2005 H. Thomas Austern Memorial Writing Competition (long papers) sponsored by the Food and Drug Law Institute.
It was not until I read "When Food is Poison" that I gained meaningful insight into the history of the Food Allergen Labeling and Consumer Protection Law. "When Food is Poison" has provided a fascinating window back in time to the then contemporaneous origins of the FALCPA's timeline, narrative and nuances. The article memorializes FALCPA's legislative intent and dealmaking, including how Grains Containing Gluten were initially classified as seriously as the other 8 major allergens and would have required labeling.
In addition, I want to acknowledge that we stand on the shoulders of giants who helped to bring the FALCPA to fruition including:
American Celiac Disease Alliance (ACDA), formerly the American Celiac Task Force (ACTF), and all of its original members.
Celiac Sprue Association (CSA) which was a champion of the FALCPA, but which went against the grain and continued to advocate for the further labeling of all Gluten-containing source ingredients, even as the legislation was being amended to make the labeling of Gluten voluntary. CSA is now part of the National Celiac Association.
Dr. Alessio Fasano, former Director of the Center for Celiac Research at the University of Maryland and whose groundbreaking research provided the Celiac community with a seat at the table during the FALCPA's journey on Capitol Hill. The American Celiac Task Force (which later became the American Celiac Disease Alliance) was formed in 2003 after the Celiac Disease prevalence study results were released by Dr. Alessio and the University of Maryland Center for Celiac Research. Current: W. Allan Walker Chair of Pediatric Gastroenterology and Nutrition Professor of Pediatrics, Harvard Medical School Professor of Nutrition, Harvard School of Public Health Chief of the Division of Pediatric Gastroenterology and Nutrition Associate Chief for Research, Department of Pediatrics MassGeneral Hospital for Children; and Director of the Center for Celiac Research and Treatment Director of the Mucosal Immunology and Biology Research Center Massachusetts General Hospital-East
Allison Herwitt, former Co-Chair, Legislative Project, American Celiac Task Force, who was one of the principal advocates for the Celiac community for FALCPA on Capitol Hill; current Chief of Staff to Senator Chris Murphy (D, CT).
Senator Ted Kennedy, who used his oratorical skills as the "Lion of the Senate" to advocate for consumer protection for those with Food Allergies and Celiac Disease.
Andrea Levario, former Co-Chair, Legislative Project, American Celiac Task Force and Executive Director, American Celiac Disease Alliance, who was one of the principal advocates for the Celiac community for FALCPA on Capitol Hill; current Senior Public Policy Advocate, Human Rights Campaign.
Rep. Nita Lowey who was one of the champions of the FALCPA and who attempted to make it ever more inclusive, including for those with Celiac Disease.
Lisa Murphy, and her son, Colin Murphy, who represented the ACTF before the House Appropriations Subcommittee on Labor, HHS, and Education in 2004, and made history by being the first people to testify on Celiac Disease before a Congressional Committee.
Eliot Spitzer, Attorney General, New York and 8 other state Attorneys General including: J. Joseph Curran Jr., Attorney General, Maryland; Jennifer M. Granholm, Attorney General, Michigan; Gay Woodhouse, Attorney General, Wyoming; Betty D. Montgomery, Attorney General, Ohio; Paul G. Summers, Attorney General, Tennessee; Richard Blumenthal, Attorney General, Connecticut; William H. Sorrell, Attorney General, Vermont; and Thomas F. Reilly, Attorney General, Massachusetts. On May 26, 2000, Mr. Spitzer wrote to the FDA, "On behalf of my office and those of eight other Attorneys General, I have enclosed a Citizen Petition seeking amendments to Food and Drug Administration rules and regulations governing food labeling, ingredient information availability and good manufacturing practice guidance. We believe that this petition provides persuasive support for FDA action in directions described in a notice to the food industry signed by Dr. Fred Shank, an FDA staffer, in 1996. Many food allergen related deaths and near-death experiences of children and adults in America can be avoided by institution of simple changes described in the petition."
Mike Taylor, former FDA Deputy Commissioner for Food and Veterinary Medicine, who told the Atlantic - "For people with celiac disease, gluten can cause serious health conditions. It's time for them to know their food is safe." Mr. Taylor also ushered in a "New Era of Gluten Free Labeling" - "For most of us, choosing a meal is not a make or break decision. Most people prepare a meal without fearing that it will endanger their health. That’s not the case with people who suffer from celiac disease. I’ve learned first-hand from talking with people with the disease how much it means to them to be able to select gluten-free foods with confidence."
In the spirit that the FALCPA has been a journey and not a destination, I want to also acknowledge that in January 2011, seven years after FALCPA was signed into law, John Forberger, a triathlete, and Jules Shepard author of “The First Year: Celiac Disease and Living Gluten-Free,” were frustrated by the lack of progress by the Federal government on the labeling to protect the Celiac community. As part of the Gluten Free Food Labeling Summit, these Gluten Free advocates developed a plan to build the world’s tallest Gluten Free cake. John and Jules rallied leaders throughout the Celiac community including Dr. Fasano, Rep. Lowey and FDA Deputy Commissioner Taylor to join them on May 4, 2011, in Washington, D.C. to kick-off Celiac Awareness Month. Their goal was to refocus attention on the way overdue labeling rules to protect the Celiac community. Their effort, along with the weight of the world's largest gluten-free cake, had an impact that resonated across the country.

Last, but certainly not least, I want to acknowledge the efforts of my daughter, Lexi Bari, who has helped me research and write, about the labeling of Gluten as a Major Food Allergen.

